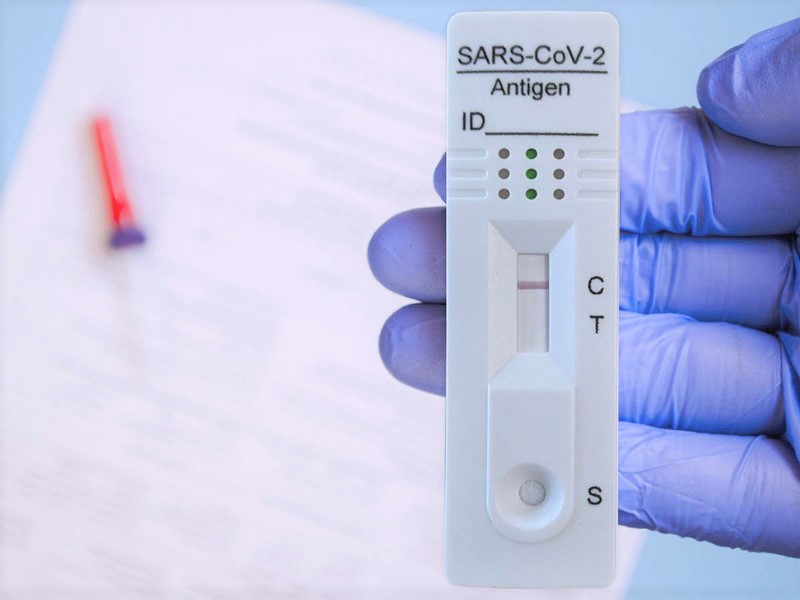
The COVID-19 Antigen Test Kit
The Westlab COVID-19 Antigen Rapid Test is a diagnostic test for the detection of SARS-CoV-2 antigens in human nasal/nasopharyngeal and oral/oropharyngeal swab specimens.
The ease of use combined with its fast results makes the Antigen Rapid Test the perfect solution for employee testing at the workplace.